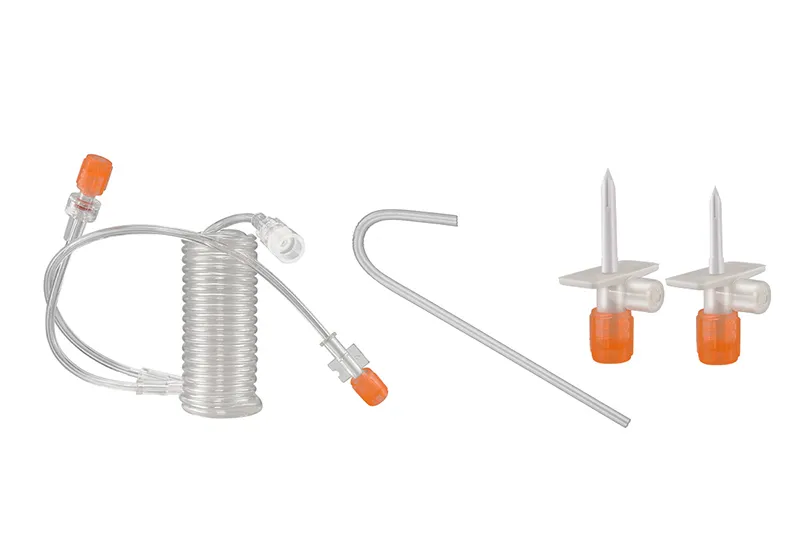
High Pressure Syringe (Contrast Injection Syringe)
- Approval: US-FDA 510 (k), EU-MDR and ISO13485
- Sterilization Method: ethylene oxide sterilization, non-toxic, non-pyrogenic
- Components: quick fill tube, CT coiled connecting/coiled Y-connecting tube, DSA connecting tube, spike
- Syringe Volume: 12ml, 20ml, 30ml, 50ml, 60ml, 65ml, 100ml, 115ml, 125ml , 130ml, 140ml, 150ml, and 200ml
- US FDA 510 (k) No.: K152906
- EU MDR No.: G10 045084 0043 Rev.00
- EU ISO13485 No.: Q5 045084 0039 Rev.01