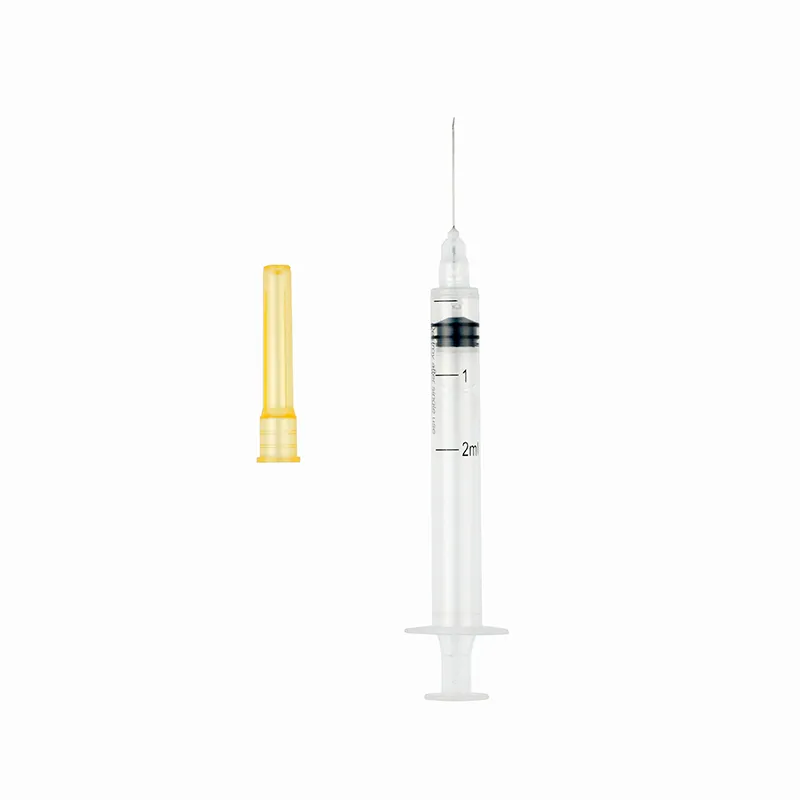
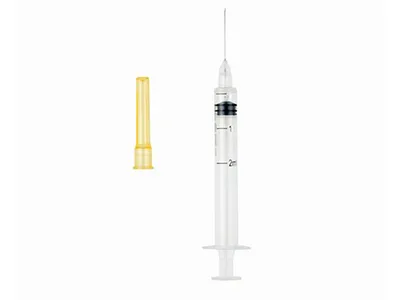
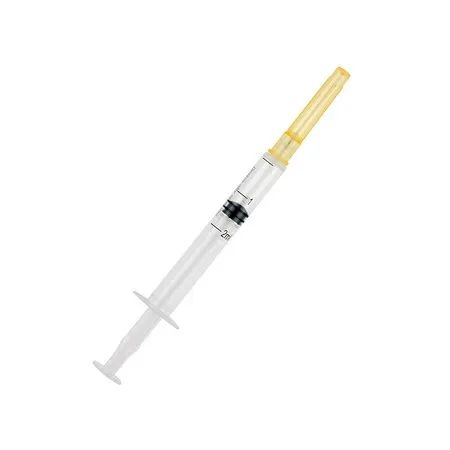
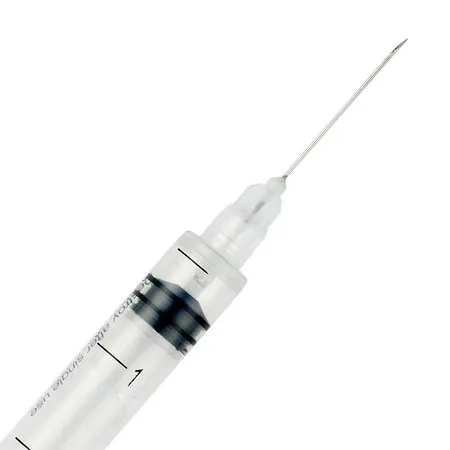
Auto-Disable Syringe (Breaking Plunger)
- Approval: EU-MDR and ISO13485, WHO certificate
- Sterilization Method: ethylene oxide sterilization, non-toxic, non-pyrogenic
- Syringe Volume: 1ml, 2ml, 3ml, 5ml, 10ml
- Jiangsu Medical Device Registration Certificate No.: 20173154044
- EU MDR No.: G10 045084 0043 Rev.00
- EU ISO13485 No.: Q5 045084 0039 Rev.01
- WHO (World Health Organization) Certificate No.: E013/041, E013/086, E013/133, E013/029, E013/083, E013/058, D013/073
- WHO PQS No.: E013/029, E013/041, E013/058, E013/073, E013/083, E013/086, E013/133, E013/136